Research overview
The copying, or replication, of DNA is one of the central processes that take place in all living organisms. Our understanding of DNA replication has made gigantic leaps forward since the discovery of the double helical form of DNA by Watson and Crick in 1953. We know many of the structures and functions of the proteins and enzymes involved. Many of these discoveries have been made by studying DNA replication in simple systems, such as viruses or bacteria. These continue to yield valuable insights, but recently, advances in the reconstitution of the yeast replisome have made it possible to gain insights into eukaryotic replication. DNA replication is carried out at very high accuracy by nanometer-scale, multi-protein complexes known as replisomes. In eukaryotic organisms such as ourselves, the replisome consists of some twenty different proteins.
Our research focuses on understanding the molecular processes that underlie eukaryotic DNA replication, with the particular aim of gaining spatiotemporal insight into their dynamics by using our single-molecule biophysical expertise in replication while integrating it with state-of-the-art molecular biology and biochemistry.

Schematic of the eukaryotic replisome. The central motor of the replisome is the CMG helicase, which unwinds the parental DNA; the polymerases are then able to add new nucleotides on the leading and lagging strands, respectively, leading to two daughter DNA molecules.
Recent works
Dynamics of replisome loading proteins
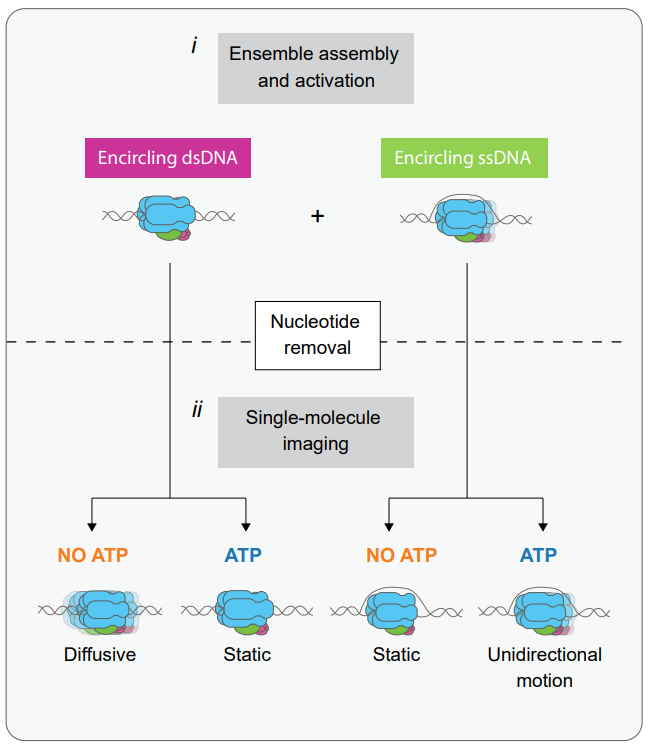
We have recently published our findings about the dynamics of the loading proteins involved in the first step of replisome assembly. The origin recognition complex (ORC) turns out to be a protein that is quite mobile on the DNA, except at the origin. Recruitment of the MCM helicase in an ORC-dependent manner can occur at different locations on the DNA, but immobile ORC-MCM complexes are also preferentially observed at the origin. When loaded onto DNA in the presence of ATP in bulk experiments and then visualized at the single-molecule level, both single and double Mcm2-7 hexamers are found on the DNA, and both exhibit similar low-level mobility. Read more about these findings here.
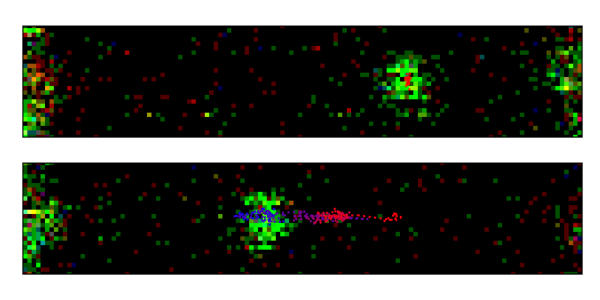
Single-molecule dynamics of ORC. Scanning confocal images of two ORC molecules, labelled in green, bound to dsDNA (not fluorescent). The dotted line connects fitted ORC positions over time (blue represents early times; red, late times). In the top panel, the ORC is static throughout the entire observation time bound to the origin of DNA replication. In the bottom panel, another ORC diffuses randomly from its initial position until it locates the origin.
Dynamics of replicative CMG helicase
Openings for students
We typically have several projects available, please contact us to find out the latest.
Researchers currently involved in these projects
- Theo van Laar
- Sara Shafiee
- Zhaowei Liu
- Daniel Ramírez Montero
- Humberto Sánchez
- Belén Solano
- Pang Yen Wang
- Francisco Palmero Moya
Current collaborators
- John Diffley Lab (Francis Crick Institute, UK)
- Alessandro Costa Lab (Francis Crick Institute, UK)
- Francesca Mattiroli Lab (Hubrecht Institute)
- Puck Knipscheer Lab (Hubrecht Institute)