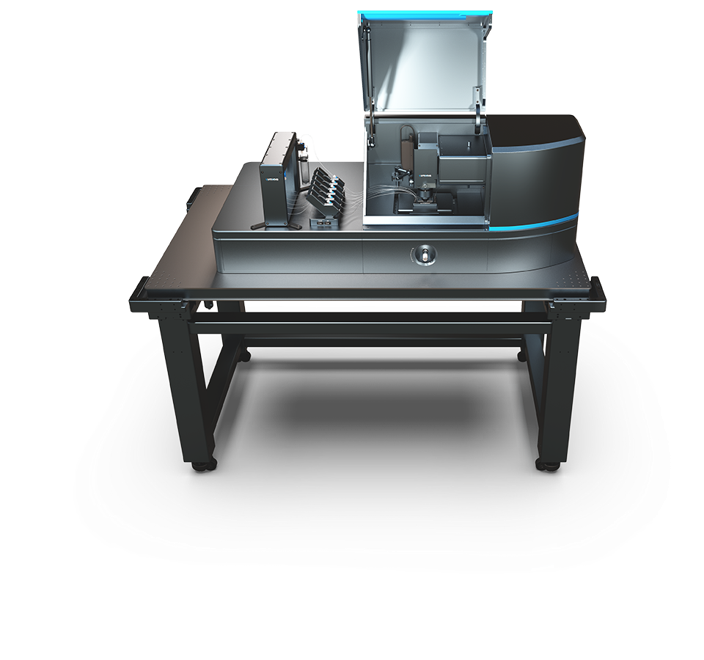
Our group brings together excellent and state-of-the-art research facilities in both biochemistry and single-molecule biophysics: a large molecular biology/biochemistry ML1 laboratory, and individual dedicated single-molecule laboratories equipped with a diverse set of home-made and commercial instruments.
ML1 biology lab
The ML1 biology lab include excellent wet lab equipment and an in-house protein purification facility. As part of the Bionanoscience department, we have also access to mass spectrometry, dynamic light scattering, and electron microscopy facilities.

Single-molecule laboratories
Our single-molecule laboratories are equipped with Lumicks C-trap and Q-trap instruments that combine optical tweezers (OT) force spectroscopy together with confocal microscopy, as well as with different state-of-the-art wide-field fluorescence microscopes that allow us to perform in vitro as well as in vivo imaging experiments. Using the latter, we perform live cell imaging, Co-localized Single-Molecule Spectroscopy (CoSMoS), and fluorescence resonance energy transfer (FRET) experiments. A collection of magnetic tweezers (MT) force spectroscopy instruments (for force, displacement and torque measurement) complete our set of cutting-edge single-molecule instrumentation.
As part of the Kavli Institute of Nanoscience Delft, we also have access to The Kavli Nanolab Imaging Centre (KNIC) which offers complementary state-of-the-art light microscopy equipment such as Structured Illumination Super Resolution Microscopy (SIM), Spinning Disk Confocal Microscopy, and Time-Correlated Single-Photon Counting Fluorescent Lifetime Imaging Microscopy (TCSPC-FLIM), as well as high-resolution cryo-electron microscopy.
Technical developments
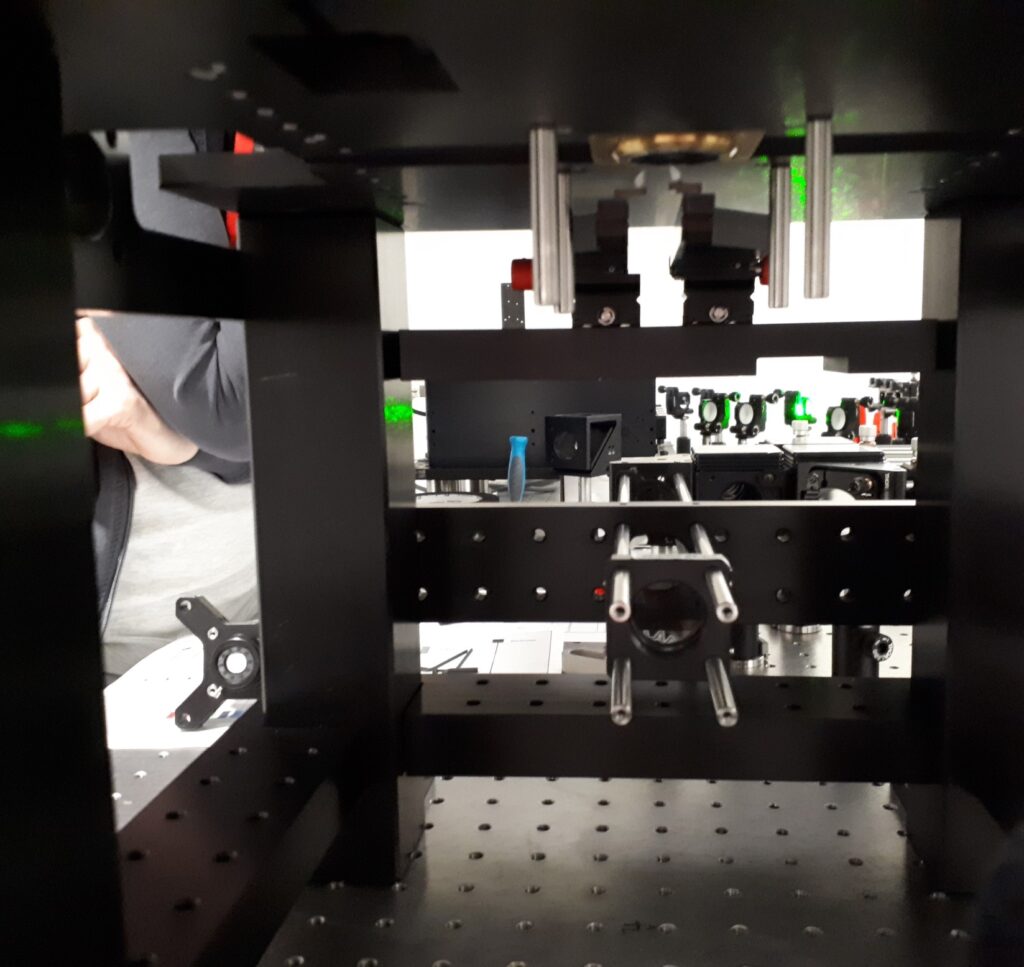
Our lab develops state-of-the-art single-molecule biophysical instruments and computational tools to ensure high data quality.
TIRF (Total Internal Reflection Fluorescence) microscopy is a powerful tool for measuring the dynamics of fluorescently-labeled single molecules. As TIRF measurements move to more complex biological systems with more fluorescent probes, the multi-band-pass dichroic that separates excitation from emission becomes limiting for the microscope’s detection efficiency. To avoid this, multicolor colocalization-based experiments can employ ‘‘micromirror’’ (mm)TIRF, which spatially separates the excitation beams from the collected emission photons. Comprehensive control of the TIR beam in mmTIRF can yield excellent signal to noise, and hence data quality, but at the price of increased optical complexity. Here, we introduce the theory behind these additional optical components and provide practical advice from our experience on the best way to set up, align, optimize, and maintain a mmTIRF instrument.

Accurate image alignment is critical in multicolor single-molecule fluorescence microscopy. Global alignment using affine transformations leaves residual errors due to the nonlinearity of the distortions, which decreases the effective field of view. Subsequent local refinement demands either large amounts of reference data and processing time or specialized imaging techniques like active stabilization. Here, we present a global alignment method, S/T polynomial decomposition, that uses sums of Zernike polynomial gradients to decompose the distortion between two images, correcting both linear and nonlinear distortions simultaneously. With minimal reference data, we gain diagnostic information about the distortion and achieve a colocalization accuracy comparable to local registration methods across the entire field of view.
